Understanding electrochemically induced surface reconstruction in oxygen evolution reaction (OER) electrocatalysts is needed to develop materials for electrochemical water splitting with improved performance and lower cost.
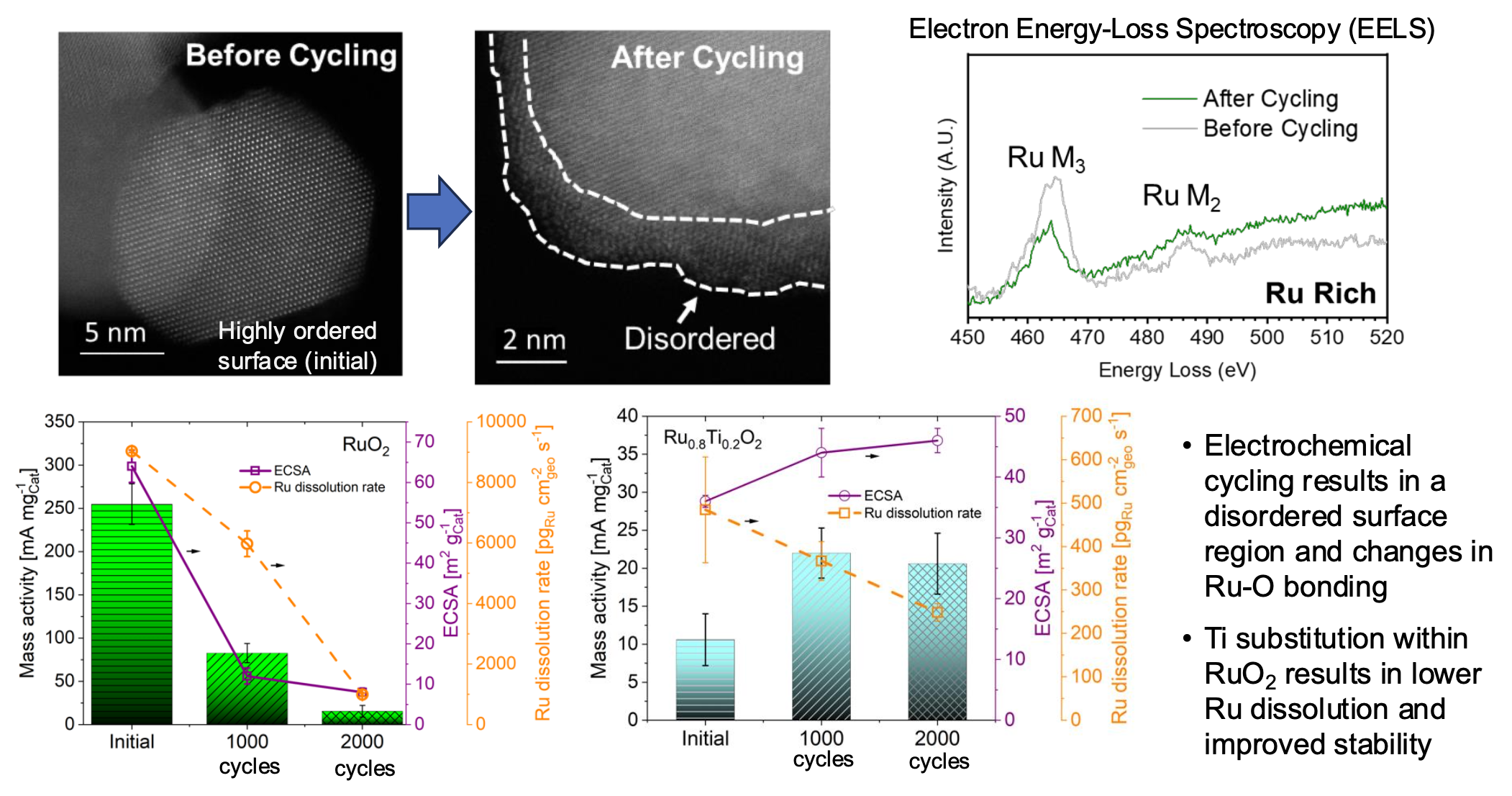
Results: We demonstrated that RuO2 and Ru0.8Ti0.2O2 undergo surface reconstruction upon cycling in OER conditions, forming a disordered surface region with changes in metal-oxygen bonding. Both RuO2 and Ru0.8Ti0.2O2 show changes in OER mass activity, electrochemical surface area (ECSA) and Ru dissolution rate with cycling; however, Ti substitution results in lower dissolution and improved stability.
Significance: Our study provides insight into the important surface atomic and electronic changes induced by metal dissolution and their effect on the oxygen evolution reaction. The multifaceted findings from our work advance our comprehension of metal dissolution dynamics and surface reconstruction and their implications for catalytic processes.
Impacts of Surface Reconstruction and Metal Dissolution on Ru1–xTixO2 Acidic Oxygen Evolution Electrocatalysts
